As of 9th Feb 2019, all actors in the pharmaceutical supply chain, including medicines manufacturers, distributors, wholesalers, pharmacies and hospitals, were due to become compliant with the Falsified Medicines Directive (FMD). The Directive’s core objective is to increase the security of the manufacturing and delivery of medicines across Europe, protecting patients and preventing falsified medicines from entering the supply chain.
For many of these organisations, FMD compliance has involved a significant re-engineering of their processes in order to receive, verify and decommission serialised medicines. And for hospital pharmacies in particular, a lack of a highly efficient workflow can potentially have a significant knock-on effect on a system that is under ever-increasing pressure.

Relying on manual processes for managing serialised medicines at an individual pack level is a labour intensive and time-consuming process which can disrupt normal operations. Instead, rather than viewing FMD from a compliance-only perspective, it can be argued that organisations should be embracing the regulation to facilitate a change in mindset throughout the medicines supply chain.
Sébastien Sliski, General Manager Supply Chain Solutions at Zetes, outlines how digitally enabled inventory processes, such as aggregation and consolidation, can help hospital pharmacies – and the pharmaceutical wholesalers who are supplying the medicines – not only meet their FMD compliance requirements in the most efficient way but yield higher efficiency gains.
Enter FMD
The Falsified Medicines Directive (FMD) is mandatory for all hospital pharmacies in Europe. However, many hospitals, pharmacies and wholesalers across Europe still remain non-compliant. While many countries introduced post-deadline stabilisation periods to help ease the transition and prevent medicine shortages, these are now coming to an end.
The main requirement mandated by the FMD is the authentication and decommissioning of medicines before dispensing to patients – including in all hospitals – together with risk-based verification and traceability of medicines at wholesaler level.
Hospital pharmacies are required to check the anti-tampering device (ATD) to ensure it is intact prior to dispensing and change the status of the pack in the National Medicines Verification System (NMVS) from “active” to “inactive supplied”. This involves scanning the 2D barcode on each pack to verify a product’s unique identifier (UI) – as applied by the manufacturer to their pack labelling - and communicating with the NMVS. Once verified, the medicine can be decommissioned. Unlike within a community pharmacy, where medicines can only be decommissioned at the point of dispense to the patient, hospital pharmacies can decommission medicines at any point after verification.
Given the importance of the pharmacy dispensing process within the overall efficiency within a hospital, and the increasing number of highly complex, niche products that require adherence to specialised distribution and dispensing models, getting this process right is critical. It is essential that hospital pharmacies can manage the verification and decommissioning process as seamlessly as possible, given the potential knock-on effect of scanning medicines at an individual pack level on the dispensing and discharge process.
Aggregation & Consolidation
The good news is that manual processes can be dispensed with. Technology is now available that consolidates the digital information of individual products’ UIs within barcode labels and allows a hospital pharmacy to decommission an entire shipment through a single scan.
This offers major time and efficiency savings; benefits that can extend further back up the supply chain to the wholesaler, third party logistics provider and manufacturer, through four simple steps:
Step 1: Aggregate
Single items are scanned at either the manufacturer or wholesaler warehouse and aggregated into boxes and / or pallets. For the wholesaler, this approach allows them to prepare shipments in advance and reduce the lead time to fulfil a dispatch, while the hospital pharmacy can choose at which level they want to decommission the products.
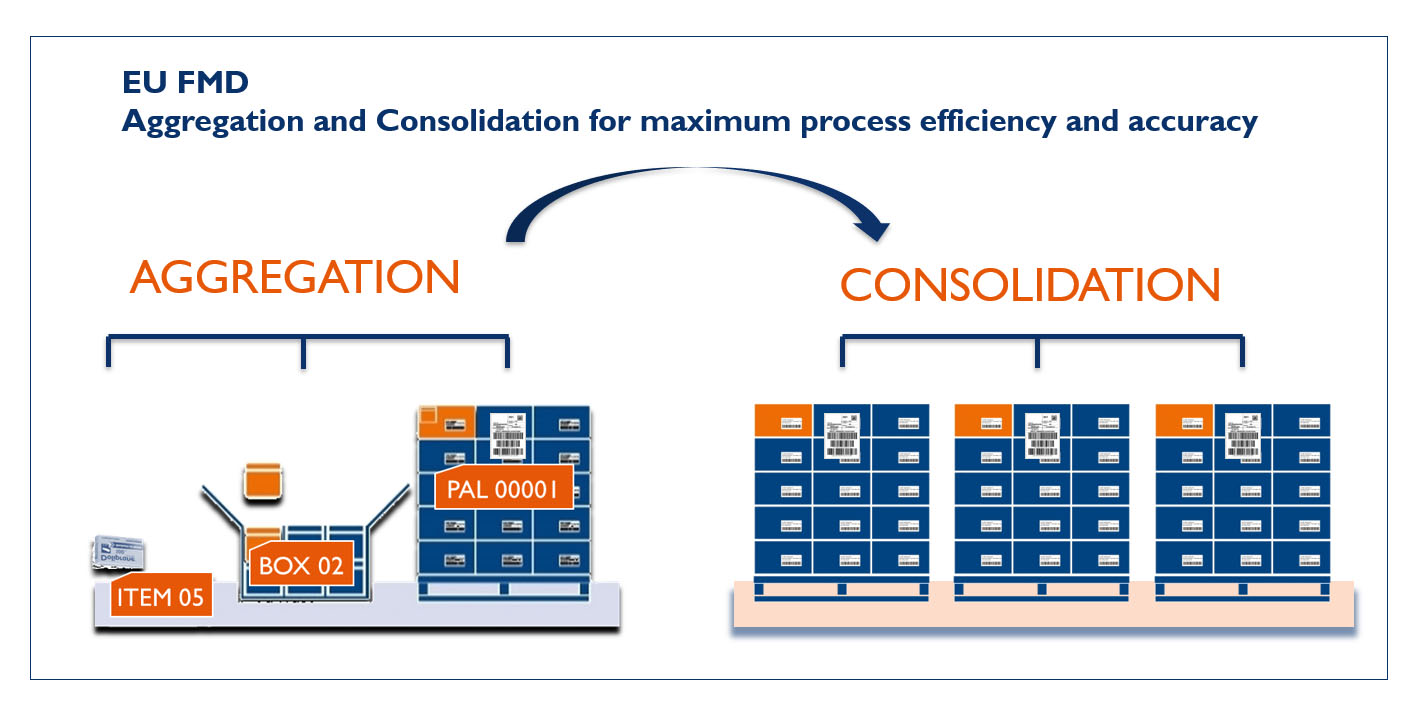
Step 2: Consolidate
Aggregated units are consolidated into a single, unique order number. This can be done at any stage of the warehousing process.
Step 3: Communicate
Aggregated units carry a barcode label with the unique order number. The dispatch process is also identified by a unique number, which can be physically placed on the transport documentation. In parallel, a digital file that contains these unique identifiers is shared with the hospital on dispatch.
Step 4: Random check & Decommission
On receipt of the shipment, the barcode numbers and digital file are available for the hospital to scan do random checking and automatically decommission the products at the specified level.
Adopting aggregation and consolidation not only addresses the compliance requirements for FMD in the most efficient way, it opens up opportunities to identify additional improvements in the pharmacy workflow and further optimise the medicines discharge process. In addition, the industry as a whole gets the chance to think beyond institutional and organisational boundaries and start to consider transparency across the broader medicines supply chain.
Conclusion
The right digital solutions can help to provide supply chain visibility to guarantee that patients everywhere have access to high-quality medicines without the risk of consuming a contaminated or counterfeit product.
Ensuring patient flow is maintained, by making sure that patients who are waiting to be discharged have timely access to their prescribed medicines, is a fundamental role of the hospital pharmacy. However, FMD goes beyond that. The right digital solutions can help to provide supply chain visibility to guarantee that patients everywhere have access to high-quality medicines without the risk of consuming a contaminated or counterfeit product.
