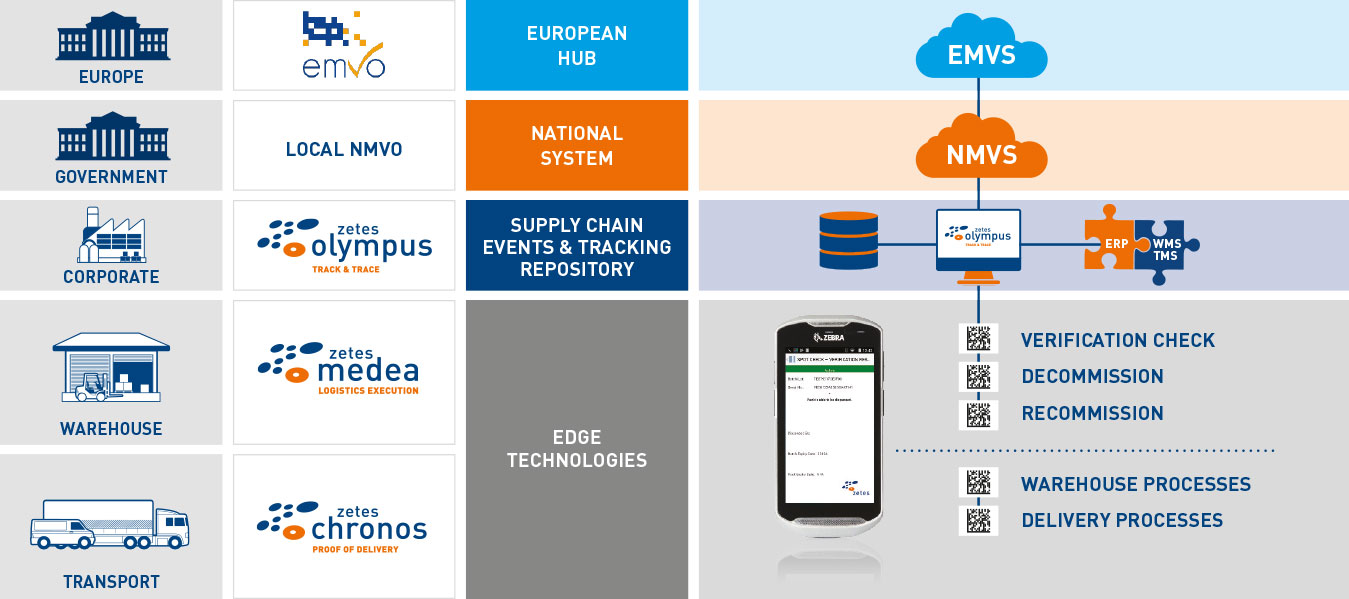
Powerful mobile application and event tracking platform
With our unique fully integrated approach you will enjoy the peace of mind you need.
FMD Mobile Application
Enables fast and easy scanning of Datamatrix codes using handheld devices to capture data and verify or decommission medicines.
1
Powerful Event and Visibility Tracking Platform
Communicates with your National Hubs (NMVS). Registers all events linked with verification and decommissioning for a period of up to 10 years.
2
Solid System Integration
Our 30+ years of supply chain systems integration ensure seamless integration with your legacy systems. Adopting our proven, risk-free approach helps you to become compliant on time.
3
Leverage your investment
With our modular approach you’ll cost effectively meet the FMD requirements and leverage your FMD investment to enjoy efficiency and traceability from the warehouse to the point of dispensing. Your FMD solution integrates seamlessly with the ZetesMedea logistics execution solution to add agility and efficiency to your warehouse operations. In addition, with our mobile proof of delivery solution ZetesChronos you can ensure timely, accurate deliveries to gain real-time delivery visibility and traceability.