What are the requirements of the Falsified Medicines Directive?
Broadly the requirements of the Directive (FMD 2011/62/EU) fall into five categories:
- Obligatory feature on the outer packaging of medicines to demonstrate identification and enable product verification
- Obligatory feature on the outer packaging of medicines to demonstrate that they have not been tampered with
- Strengthened requirements for the inspection of the manufacturers of active pharmaceutical ingredients
- The obligation for manufacturers and distributors to report any suspicion of falsified medicines
- An obligatory logo that must be placed on the websites of legally operating online pharmacies, with a link to official national registers.
An end-to-end FMD compliance solution
Process and technology integration experts for the warehouse.
Zetes is currently helping pharmaceutical manufacturers and wholesalers across Europe to cost-effectively ensure compliance with the Falsified Medicine Directive. Our fully integrated, packaged solution for wholesalers incorporates:
- Risk-based serial number verification
- Serial number Decommissioning and Recommissioning
- Article 23 Decommissioning
- Publishing to local National Medicines Verification Systems (NMVS)
- Reporting and event history
- Where required, additional warehouse process optimisation & track & trace enablement
An integrated solution to ensure compliance
The Falsified Medicines Directive (FMD) 2011/62/EU came into full force on 9th February 2019.
As a wholesaler, to ensure compliance you will need to be able to conduct risk-based verification, decommissioning or recommissioning of serialised packs. We help you ensure compliance by empowering warehouse operations with 2D data matrix barcode scanning and decoding capability with a direct connection to your country’s national verification system (NMVO). Discover our cost-effective, integrated solution that works with your existing systems and processes.
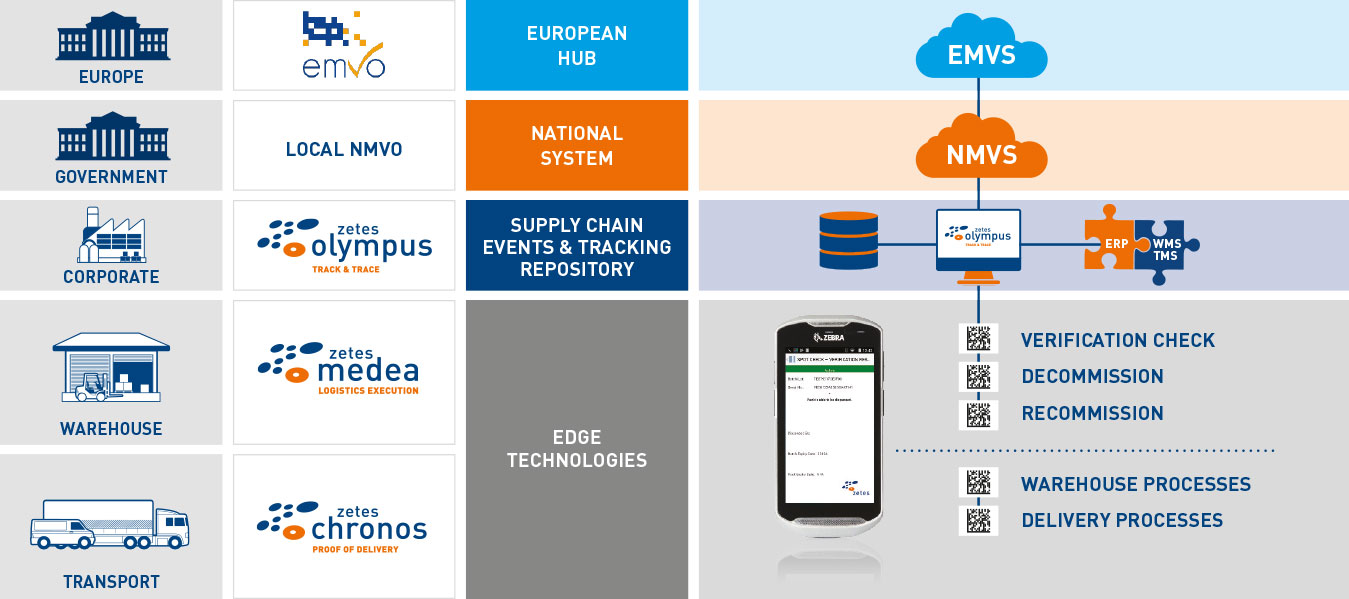
Powerful mobile application and event tracking platform
With our unique fully integrated approach you will enjoy the peace of mind you need.
FMD Mobile Application
Enables fast and easy scanning of Datamatrix codes using handheld devices to capture data and verify or decommission medicines.
Powerful Event and Visibility Tracking Platform
Communicates with your National Hubs (NMVS). Registers all events linked with verification and decommissioning for a period of up to 10 years.
Solid System Integration
Our 30+ years of supply chain systems integration ensure seamless integration with your legacy systems. Adopting our proven, risk-free approach helps you to become compliant on time.
Leverage your investment
With our modular approach you’ll cost effectively meet the FMD requirements and leverage your FMD investment to enjoy efficiency and traceability from the warehouse to the point of dispensing. Your FMD solution integrates seamlessly with the ZetesMedea logistics execution solution to add agility and efficiency to your warehouse operations. In addition, with our mobile proof of delivery solution ZetesChronos you can ensure timely, accurate deliveries to gain real-time delivery visibility and traceability.
Who do we help?
“There is a concern that in order to introduce FMD compliance into the warehouse, a costly ‘rip and replace’ approach is required. This doesn’t have to be the case.”
Christian Taylor - Serialisation Expert at Zetes
Read the articleWant more info or request a free, non-binding consultancy meeting
Contact UsHow can we help you?
- Pharmaceutical manufacturers and CMO's: Our ZetesAtlas pharmaceutical serialization solution helps you ensure compliance with the directive. We offer you a proven approach and a single point of contact to mitigate risk and ensure swift project implementation so you will be ready on time and within budget.
- Wholesalers and logistics service providers: Our FMD compliance solution empowers your warehouse with the capability to conduct risk-based verification, decommissioning or recommissioning of serialised packs.
