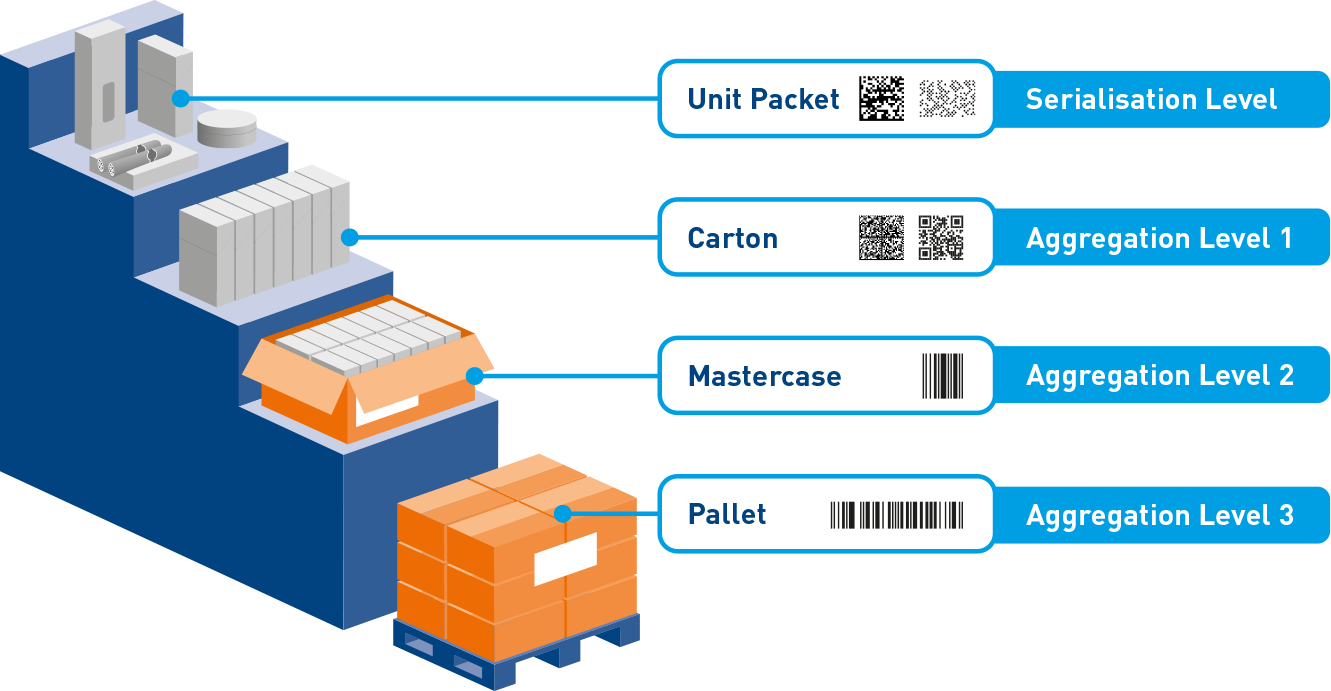
The key features of our serialisation system
- Ensures Operational Equipment Efficiency (OEE).
- Provides efficient and centralised line management.
- The technology works independently.
- Creates a serialisation audit trail.
- Generates serial numbers or allocates them from an external source.
- Provides validation with a control, check and verify process.
- Establishes e-Pedigree.